About Client
Industry: Medical Devices
Product: Microfluidic Chip for devices of advanced diagnostics
Location: Scotland, UK
Why Xu Feng
Customer challenges: The unexpected surge in demand for microfluidic chips during the pandemic left the industry scrambling to adapt. As post-pandemic demand spiked, many companies faced operational challenges. For example, FMS was striving to integrate embossing into their automated production line to enhance efficiency. However, maintaining precise hole alignment during die-cutting has proven difficult, prompting them to explore alternative manufacturing solutions.
Capabilities Leveraged: Etching, CNC, Plastic injection molding, Metal Sheet Fabrication.
Result: Four trial molds were successfully designed and produced to prototype various texture plates, achieving precise hole alignment for the microfluidic chips.


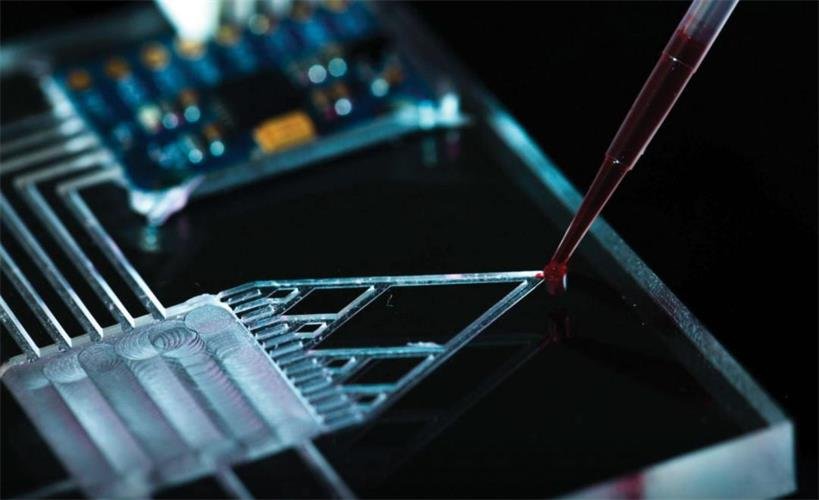
Unexpected manufacturing challenges
FMS is revolutionizing disease prevention and treatment with cutting-edge diagnostic technologies. Founded to simplify preventive and therapeutic diagnosis worldwide, FMS focuses on routine health checks, epidemiology, and advanced cardiovascular interventions.
Aim to Using just a small blood sample, FMS plan to deliver comprehensive diagnostics and treatment planning. This enables minimally invasive procedures, empowering doctors to act swiftly and effectively.
“Our mission is to provide individuals with an objective understanding of their health, enabling doctors to perform precise evaluations and timely interventions,” says Kam Ghofrani, CEO of FMS. “Prevention is more powerful than treatment. Today, the economic burden of managing diseases is overwhelming. Often, delays in testing result in missed opportunities for early, life-saving care.”
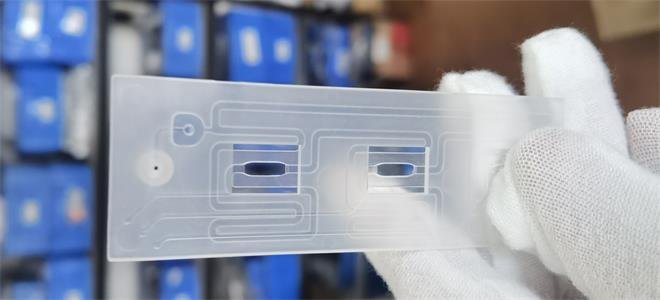

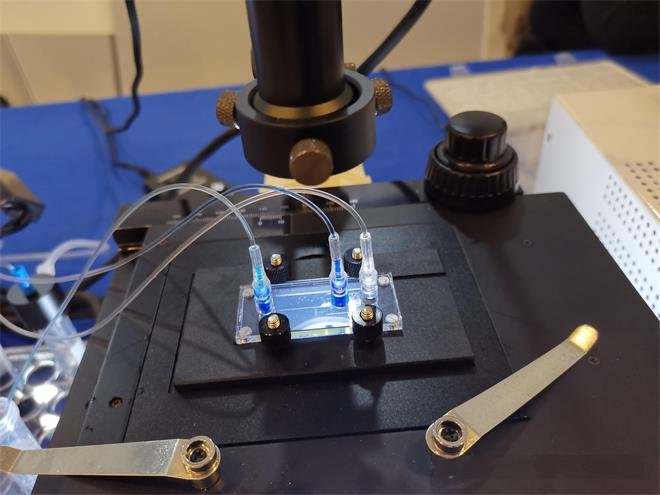
“From the start, we recognized the need to deploy several next-generation technologies simultaneously, but conventional development costs could hinder our progress.” Kam Said.
Kam’s original company was a small medical equipment manufacturer, Initially constrained by limited personnel and equipment, the company’s output was very low. Until this point, they’d been successful using their in-house manufacturing capabilities with a relatively small external suppliers. However, this setup was not scalable enough to meet rising customer demands, especially against the backdrop of pandemic-induced supply shortages. This situation put the company’s financials at risk.
If you are operating a medical device company, this kind of problem might sound all too familiar. You could try to maximize in-house production, running 24-7 to try to catch up, find more 2 and 3 suppliers to help, You might even consider heavily investing in procurement specialists to spearhead these efforts, Yet, these solutions are often slow to implement and may not achieve the necessary quality and speed to overcome production challenges, particularly within the stringent regulations of the medical device industry. And it’s not going to get any easier.
“From the start, we recognized the need to deploy several next-generation technologies simultaneously, but conventional development costs could hinder our progress.” Kam Said, “Recent studies show that the average cost to bring a single new Class II medical device from initial concept to MDR regulations before market launch is from a few million euros to over 10 million euros.”
The Solution from Xu Feng
Choosing A Faster, Efficiency Path to Market
After interfacing with Xu Feng, the FMS team realized that they could speed up the workflow and thus significantly accelerate the product development cycle time. For example, by submitting design drawings, you can expect rapid delivery from Xu Feng- often within a week. Even at the conceptual stage, XuFeng is committed to seamless communication and resource integration, swiftly turning your initial ideas into tangible prototypes.
Andrew Mashrequi, Chief Design Engineer at FMS, says this is a huge step forward. “Working with XuFeng has been transformative for our project momentum. We used to pause and disconnect from projects, losing valuable time. Now, we maintain continuous progress and bring innovations to market faster than ever.”

“So, we decided to adopt a new strategy by collaborating with Xu Feng, leveraging its manufacturing facilities and extensive network partners to match with our extremely talented, but lean and mean internal design team. It enabled us to save millions of dollars in infrastructure, labour, and time costs compared to traditional development and supply chain strategies.”

Reducing Cycles From Years & Months
Instead of overhauling their supply chain alone, FMS partnered with Xu Feng to tap into an agile, ready-made network. Xu Feng’s comprehensive workshop features CNC machining, injection molding, and sheet metal fabrication capabilities, crucial for crafting PCB trays and detector housings. Additionally, Xu Feng collaborates with a specialized partner offering precise etching services for microfluidic chips, achieving channel depths between 5-120 μm. The flexible combination provides series development and optimization as well as end-to-end supply chain support.
- From April 2022 – January 2023, Xu Feng skillfully machined four distinct models of small-sized aluminum products, presenting two high-volume production strategies to enhance cost-efficiency and productivity.
- Between March 2023 and August 2024, Xu Feng completed the drawing processing and sheet metal fabrication for four iterations of another model. FMS tested these prototypes, garnering promising experimental results. In a strategic move to further reduce costs by 30% while adhering to quality standards, Xu Feng recommended a CNC combined with a Stamping method for mass production
- As of May 2023, Xu Feng has successfully designed and manufactured an experimental injection mold, undergone four mold modifications, and tested five materials across 15 patterns of microfluidic panels. In pursuit of scaling production efficiently, Xu Feng introduced the T-mold production method, poised to significantly streamline future mass production efforts.
By integrating with Xu Feng’s scalable and flexible supply chain, the product was developed in just over two years, compared to 5 years using the existing supply chain.

Kam said “They’re great about answering our questions and giving us immediate attention and feedback when we have issues.”


A Trusted Partner for Seamless Communication
“Periodically, we engage in video conferences with the folks at Xu Feng, and they bring in the people we need -whether it’s a salesperson, a technician, an engineer, or a molding specialist. Since testing each new model is a time-intensive process, we emphasize the need for process adaptability to seamlessly transition from prototyping, through small batch production, to full-scale manufacturing, all while maintaining consistent part quality.
This quick turnaround feedback helps avoid problems, shorten cycle times between design iterations, and smooth the transition from prototyping to production. “We’ve actually learned a lot from the people at Xu Feng, which has helped us a lot,” said Dr. Mashrequi. “For our team, integrating design concepts with actual production scenarios is crucial to effectively translate ideas into practical applications.
“In my experience, successful medical device companies must accelerate NPIs to gain access to markets
But in many cases, companies lack the resilient supply chain, technology, and quality support needed to bring products to market. Fortunately, we have found a flexible partner who can deliver.”
Scaling While Staying Lean
This level of communication and engagement fosters a trusting relationship that fully leverages the potential of FMS. Prior to this, Kam had founded several companies. Now, having experienced the efficacy of agile, ready-made partners, he wouldn’t choose any other way.
“Typically, at previous companies, we handled things differently. We kept all our new product development in-house because we wanted to control our schedules and keep the confidentiality of our technology. However, maintaining everything in-house required a lot of facilities, capitals, and cost of management,” Kam explains. “Even in the early stages of prototype development, we were burning through hundreds of thousands of dollars each month.”
With the assistance of Xu Feng, FMS has significantly reduced the time and money spent on building and maintaining infrastructure, allowing them to allocate resources where they matter most— developing the technology. The economic benefits of accelerating time-to-market for these new products are clear and it coincides with FMS’s mission to make diagnosis and treatment more accessible.
“My previous company took at least five years to bring a new product to market,” said Dr. Mashrequi. “Xu Feng has made it possible to shorten the time to market for our first few models by 3 years.”
Kam also agrees, “Working with Xu Feng has allowed us to keep our team lean. We are close to commercializing this breakthrough medical diagnostic, and we are confident that we will achieve a competitive position in the industry.”

